Modeling of Outflow Boundary Conditions Taking Account of the Effect of Peripheral Vessel Network for Hemodynamic Simulation
In order to achieve an in vivo simulation of living organisms, it is important to apply appropriate physiological conditions such as physical properties, models, and boundary conditions. Generally, the numerical simulation using a patient-specific model is conducted for a localized region near the research target. Although the analysis region is only a part of the circulatory system, the simulation has to include the effects from the entire circulatory system (such as pulsation by heart or resistance due to bifurcation of vessels).
Therefore, we focus on modeling of proper outflow boundary conditions taking account of the effects of peripheral vessel network (small arteries, arterioles and capillaries), which can not be resolved in medical images, to examine hemodynamics of the cerebral arterial circle of Willis (Fig.1), in which includes all preferential locations of the cerebral aneurysms using our Image-Based Modeling and Simulation System.
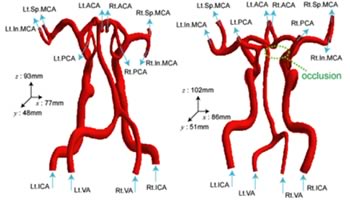
Fig.1: Computational models of the arterial circle of Willis (left: without occlusion, right: with occlusion)
Figures 2 and 3 show the distribution of flow rates at each vessel of the arterial circle of Willis with or without occlusion, respectively. The values are at the peak of systole.
In case of the arterial circle of Willis without occlusion, blood flows from anterior to posterior with free-stream boundary condition (Fig.2, left). On the other hand, it does with the multi-scale boundary condition from posterior to anterior although with the inlet flow rates are higher in the anterior than in the posterior (Fig.2, right).
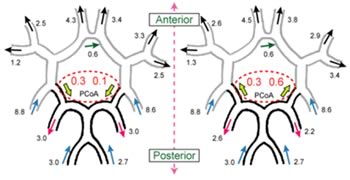
Fig.2: Distribution of flow rates (left: free stream boundary condition, right: the multi-scale outflow boundary condition)
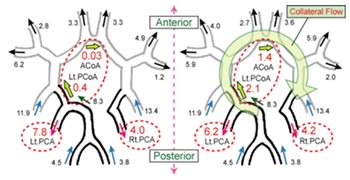
Fig.3: Distribution of flow rates (left: free stream boundary condition, right: the multi-scale outflow boundary condition)
In case of the arterial circle of Willis with occlusion, all of flow from vertebral artery (VA) tends to go into left side of arterial network. With free-stream boundary condition (Fig.3, left), the flow rates at left posterior cerebral artery (Lt.PCA) are much higher than that at right posterior cerebral artery (Rt.PCA). On the other hand, with the multi-scale boundary condition (Fig.3, right), the flow rates from left posterior communicating artery (Lt.PCoA) to anterior communicating artery (ACoA) increase. As a result, the difference of flow rates between left and right posterior cerebral arteries becomes small. This phenomenon seen in case of occlusion is called collateral flow such that blood flow bypasses through another blood vessel.
From these results, the present method shows a significant difference in flow rate of each artery and improvement in flow distribution and direction.
References
Oshima, M., Tokuda, S., Unemura, T., Sugiyama, S.: “Numerical simulation of blood flow in the circle of Willis with outflow boundary conditions using a one-dimensional model”, Proceedings of 5th World Congress of Biomechanics (CD-ROM), 2006.
Tokuda, S., Sugiyama, S., Unemura, T., Oshima, M.: “Modeling of Outflow Boundary Conditions Based on Peripheral Vessel Network for Hemodynamic Simulation”, Proceedings of JSME Mechanical Engineering Congress, 2006 Japan, Vol.6, pp.53-54, 2006 (in Japanese).